Abstract
Introduction: The acquisition of cytogenetic aberrations (CA) and/or of molecular mutations (clonal evolution, CE) is known to be associated with poor outcome in pts with myelodysplastic syndromes (MDS) (Jabbour et al, Am J Hematol, 2013; Cevik et al, abstract, Onkologie, 2013). The aim of our study was to identify additional cases with CE by taking cryptic CA, which cannot be detected by conventional chromosomal banding analysis (CCB), and their interaction with molecular mutations, into account.
Methods: We retrospectively reviewed 103 pts with proven MDS (10x RCUD, 40x RCMD, 10x RAEB-1, 16x RAEB-2, 11x sAML after MDS, 3x MDS-U, 7x CMML, 6x MDS with unknown subtype). Karyotype from CCB and molecular karyotype from SNP-array analysis (SNP-A) were available for all pts. SNP-A and fluorescence in situ hybridization (FISH) analyses were used to detect cryptic CA. For 57 pts results from mutational analysis from a 17-genes Sanger and/or a 54-genes next generation sequencing panel were included. Genetic follow-up and survival data were available for 57 pts, 22 pts were genetically analyzed under treatment with disease modifying therapies. Genetic follow-up comprised analysis of bone marrow and/or frequent sequential genetic monitoring of CD34+ peripheral blood cells.
Results: CA were detected at diagnosis in 65/103 pts (63%). By CCB (aberrant in 55/103 pts; 53%) the median number of CA was 1 (range: 0-4). One to 6 (median 1) cryptic CA were detected in 31/103 (30%) pts, 10/31 pts (32%) showed a normal karyotype in CCB. The cryptic CA included cryptic copy number variations (CNV) in 12 pts and CN neutral losses of heterozygosity (CN-LOH) in 19 pts as the sole cryptic CA (14x) or in addition to cryptic CNVs (5x). In 12 pts with CN-LOHs we could identify a homozygous molecular mutation or a cryptic deletion in the region of a CN-LOH. Genes mutated in the region of a CN-LOH were DNMT3A (2p23.3, 2 pts), EZH2 (7q36.1, 2 pts), CBL (11q23.3, 2 pts), RUNX1 (21q22.12, 2 pts), TET2 (4q24), IKZF1 (7p12.2), TP53 (17p13.1), and SRSF2 (17q25.1). The formation of this phenomenon was observed in consecutive samples of 3 pts.
From the 57 pts with follow-up data available, 8 showed manifestations of antecedent CE at first diagnosis (2x sub-clones; 6x homozygous mutations in CN-LOHs), from which 7 showed further genetic progression during the course of the disease. CE after first diagnosis was also observed in 20 of the 49 other pts with available follow-up data. The new aberrations of the biggest clone at first CE after diagnosis were partial or complete LOH of chromosome (chr) 7 (6 pts), trisomy 8 (5 pts), chr 21 abnormalities (2 pts), chr 12 abnormalities (2 pts), CN-LOH in 4q, 11p-, 20q-, -X, an additional marker chromosome, tetraploid cells, and mutations in ASXL1 (2 pts), CDKN2A, ETV6, IDH1, and JAK2 . Further steps of CE in these pts are currently being evaluated.
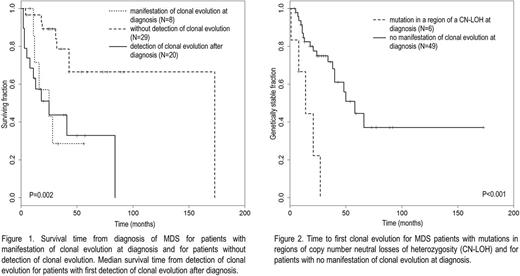
Median survival time from detection of the first CE after diagnosis (25 months, 12/20 deaths) did not differ significantly from median survival time from first diagnosis for pts with signs of antecedent CE at first diagnosis (25 months, 5/8 deaths, P=0.92, n.s.). Median survival time of pts without detection of CE was significantly longer (173 months, 8/29 deaths, P=0.005 and <0.001, Fig. 1). 5/6 pts with mutations in regions of CN-LOHs at diagnosis and available follow-up data showed further CE. 19/49 (39%) pts without any signs of antecedent CE at diagnosis showed CE during the course of the disease. Time to first CE after diagnosis was significantly shorter in the first group compared to the second one (14 vs. 58 months, P<0.001, Fig. 2).
Conclusions: In our cohort of MDS pts the frequency of cryptic CA was 30%. A homozygous mutation in a region of CN-LOH, which indicates antecedent CE, was detected in 12% of pts. The presence of such evolutionary patterns at first diagnosis might be indicative of a genetically instable disease as these pts had a shorter survival time and a shorter time to further genetic progression. There is a clear tendency that cytogenetic LOHs preferentially affect regions with molecularly mutated genes, resulting in homozygous mutations. The development of these patterns seems to be an important step towards progression. Our data imply that the relevance of cryptic CA for CE and disease progression in MDS has been underestimated as yet. Cryptic CA should be taken into account for developing prediction models for clinical progress and for improving individualized patients care.
Al-Ali: Gilead: Consultancy, Honoraria; Alexion: Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Metz: Celgene: Other: study projects, advanced training support; Novartis: Consultancy, Other: study projects, advanced training support. Germing: Janssen: Honoraria; Novartis: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Platzbecker: Janssen: Consultancy, Honoraria, Research Funding; Acceleron: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Research Funding; Celgene: Consultancy, Honoraria, Research Funding. Haase: Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal